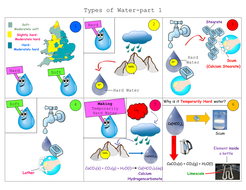
What Is Hard Water In Chemistry. Ferrous iron may also be present; oxidized to the ferric form, it appears as a reddish brown stain on washed fabrics and enameled surfaces. Hard water (also referred to as water hardness) is a very common characteristic associated with water which contains dissolved chemical compounds of magnesium and calcium, and sometimes, certain other trivalent and divalent metallic elements. hard waterfrom the municipal water supply.
Hard water: The water with naturally present minerals like magnesium and calcium with detectable amount is called hard water.
General hardness (GH) refers to the dissolved concentration of magnesium and calcium ions.
People often ask, "what is the best water filter for hard water?" Hard water has dissolved minerals in it, which can leave unsightly spots on hard surfaces like windows, tables, floors, silverware, dishes and glassware. Water softening systems work by reducing. Note: GH, KH and pH form the Bermuda's Triangle of water chemistry. And here is the problem for brewers: recall that I said earlier that good brewing water should be moderately hard. Ferrous iron may also be present; oxidized to the ferric form, it appears as a reddish brown stain on washed fabrics and enameled surfaces. Please note that these assume some.
FuseSchoolLearn the basics about hard and soft water, the differences between these and where to fi. Chemical water analysis is carried out on water used in industrial processes, on waste-water stream, on rivers and stream, on rainfall and on the sea. Hard water can be softened by adding washing soda (sodium carbonate) which removes the calcium ions in a precipitation reaction. These ions react with soap, making it difficult to form a lather and producing scum. Layers of Learning has hands-on experiments in every unit of this family-friendly curriculum. Hard water is water containing high amounts of mineral ions.
And here is the problem for brewers: recall that I said earlier that good brewing water should be moderately hard. Soft water: It is treated water. Note water may contain minerals and yet not be considered hard, by this definition.