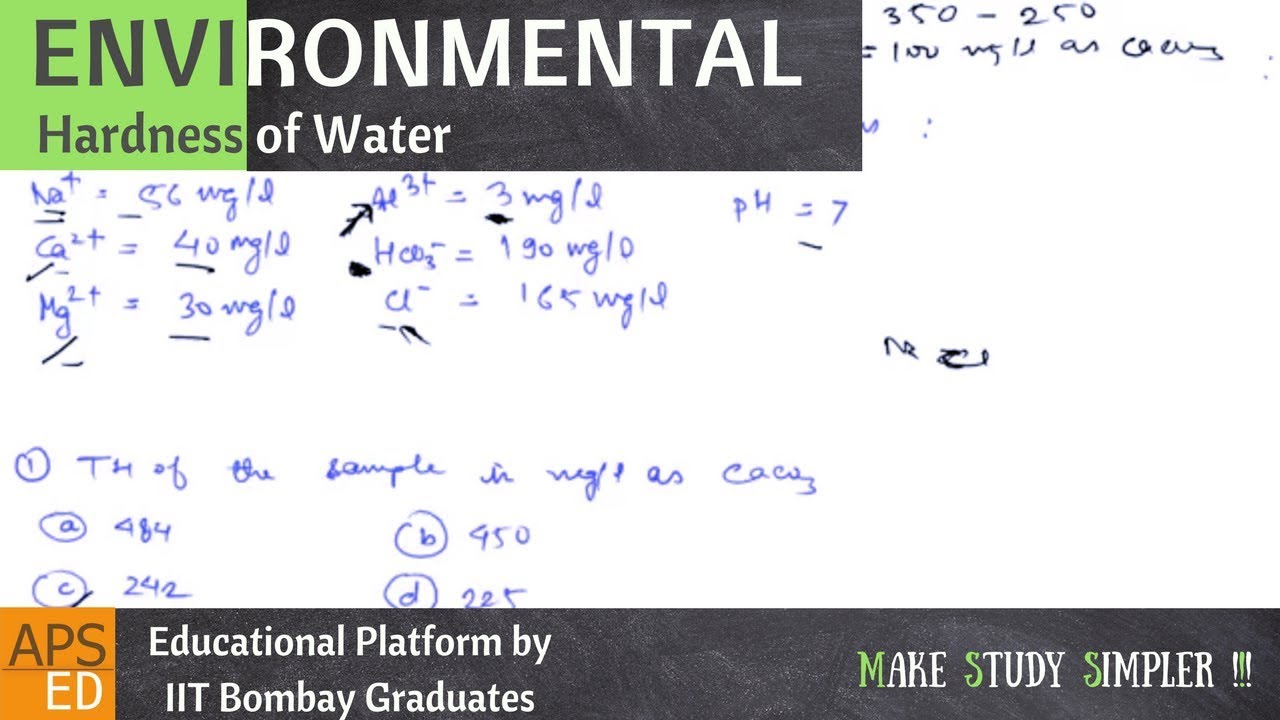
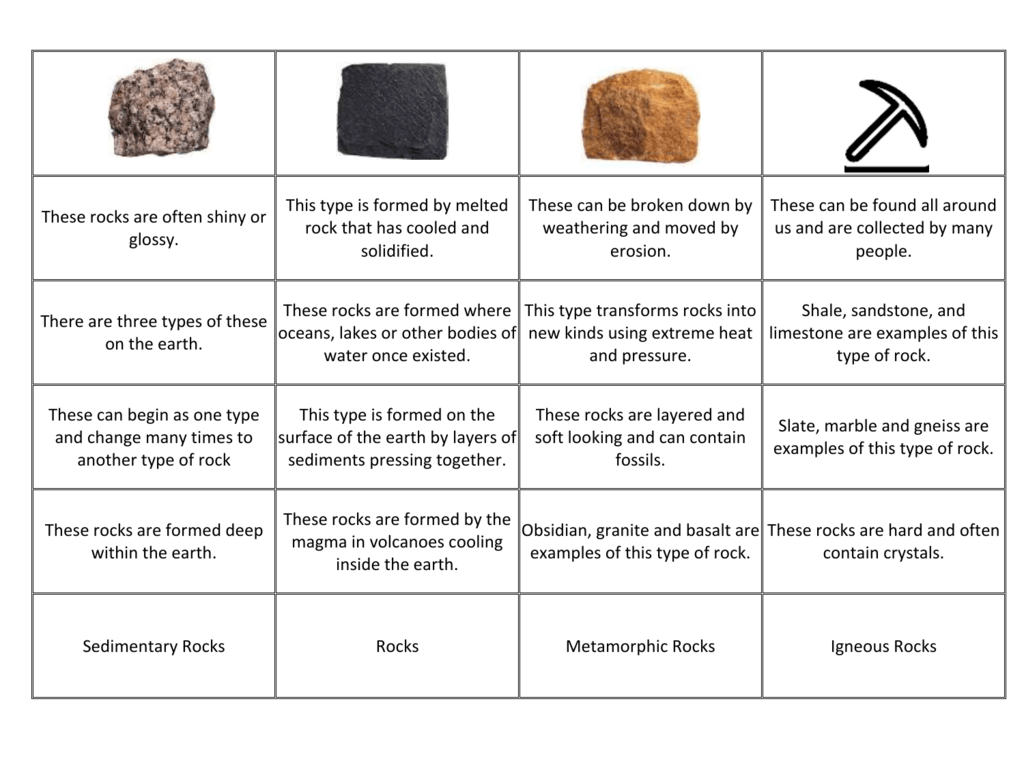
3 Example Of Hard Water. For example, water from an underground aquifer will be harder than water from a river or lake. Water hardness that is caused by calcium bicarbonate is known as temporary, because boiling converts the bicarbonate to the insoluble.
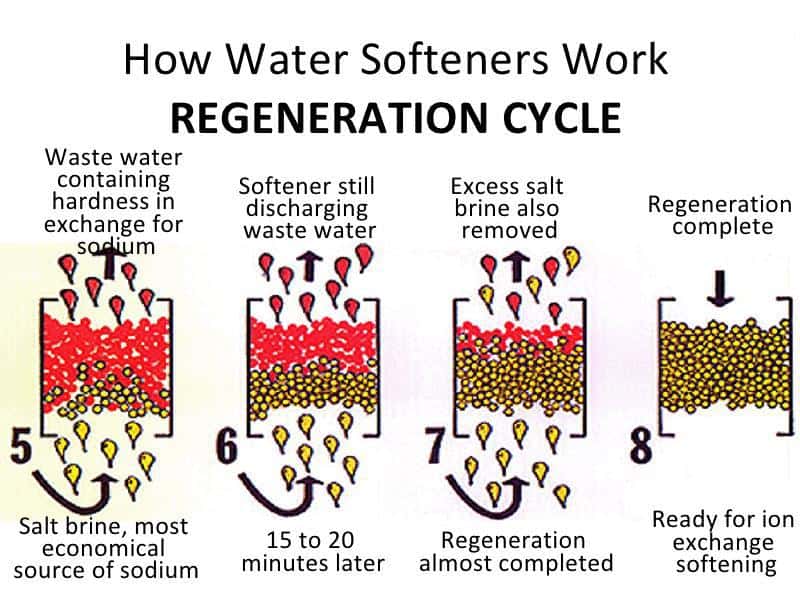
White scale buildup on plumbing fixtures is often a good indicator of the presence of hard water.
Softened water is gentler than hard water, so is easier on appliances and pipes.
Water is a great solvent for calcium and magnesium, so if the minerals are present in the soil around a water-supply well, hard water may be delivered to homes. Solutions containing the group two metal ions are called "hard" water. Students understand the terms: micelle, hard water, soft water, temporary hardness, and permanent hardness. The water, which does not lather with soap, is called hard water. The scale may have a whitish color, and likely does not wipe away easily. Ferrous iron may also be present; oxidized to the ferric form, it appears as a reddish brown stain on washed fabrics and enameled surfaces.
Another unsightly issue is the appearance of those white, hard water spots. Water hardness that is caused by calcium bicarbonate is known as temporary, because boiling converts the bicarbonate to the insoluble. This is because soap molecules are negatively charged on one end to help the molecule dissolve in water. Hard water is a term that denotes water having a very high mineral content (the term is the opposite of 'soft water'). As the mineral content in the water increases, the water is considered "harder". The scale may have a whitish color, and likely does not wipe away easily.
This is because soap molecules are negatively charged on one end to help the molecule dissolve in water. Hard water occurs under the natural condition when the water moves through rocks like limestone and chalk that consist of minerals like calcium carbonate and magnesium carbonate. Ferrous iron may also be present; oxidized to the ferric form, it appears as a reddish brown stain on washed fabrics and enameled surfaces.